world health news
March 23, 2020
We had many questions on treatment for COVID 19 so here is breakdown of the most up to date information as of today’s post:
Outpatient: Patients who are stable and breathing comfortably on room air, send them home. Advise them to self-isolate (stay in separate room, wear mask when around others, frequently wipe high-touch surfaces, don’t share household items with others), use Acetaminophen for fever and myalgia, and keep themselves hydrated. Give them a phone number to call if their condition deteriorates.
Inpatient: Patients with moderate to severe symptoms should be hospitalized for observation and treament.
Isolation: All patients with suspected or confirmed COVID-19 should be placed in Modified droplet precautions which include gown, gloves, mask, and eye shield. Airborne precautions (that include use of N-95 masks or PAPR) or are recommended with aerosol generating procedures such as airway suctioning, intubation, sputum induction, endoscopy, etc.
Contact your institutional infection control department for further guidance as details may vary based on available resources.
Supportive care: Cornerstone of therapy at this time. This may include oxygen, mechanical ventilation, ECMO, electrolyte management, etc. Lots of helpful infomation at Internet Book of Critical Care.
Anti-viral medications:
For now, use of anti-viral medications should be reserved for patients requiring hospitalziation.
Chloroquine: Safe and cheap. Both chloroquine and hydroxychloroquine are effective in limiting the replication of SARS-CoV-2 in-vitro. Proposed mechanisms include alternation in the ACE-2 receptor configuration on cell surface (blocking viral entry in cells), interference with acidification in phagolysosome (blocking viral replication), and immunomodulation by interfering with Toll-like receptor (blocking cytokine storm).
Some early promising data showing that chloroquine use improves lung imaging, shortens time to viral clearance, and shortens disease course.
In another open-label study (n=36), use of hydroxychloroquine (200 mg t.i.d. x 10 days) was associated with a higher rate of undetectable SARS-CoV-2 RNA on nasopharyngeal specimens at day 6 compared with standard of care (70 versus 12.5 percent). It this study, it was combined with azithromycin to prevent/treat superimposed bacterial pneumonia.
See this systematic review for more information.
Based on in-vitro data and pharmacokinetics, a loading dose of 400 mg b.i.d. of hydroxychloroquine sulfate given orally, followed by a maintenance dose of 200 mg b.i.d. x 5 days has been recommended (chloroquine phosphate dose is 500-mg b.i.d. x 10 days)
Check G6PD level before chloroquine use (not needed for hydroxychloroquine)
Can it be used as prophylaxis? See below in Prevention section.
Hydroxychloroquine PLUS azithromycin combination: An open-label non randomized clinical trial of 36 patients showed that hydroxychloroquine was associated with significantl viral load reduction/disappearance in COVID-19 patients and its effect was reinforced by azithromycin.
Remdesivir: A broad-spectrum antiviral drug. Remdesivir was initially developed to treat Ebola but has activity against mutiple RNA viruses such as RSV and the coronaviruses (including MERS and SARS viruses). It was initially available for compassionate use but is now only available through clinical trials. Currently being investigated for severe disease. However, best time to use it may be early in disease when most of the damage is being caused by direct viral replication and CPE and not late stages when cytokine storm is responsible for organ dysfunction.
Tocilizumab: A humanized monoclonal antibody against the interleukin-6 receptor. Tocilizumab is currently used for various auto-immune diseases including RA and cytokine release syndrome, a side effect of CAR-T celltherapies. It is expected to reduce lung and vascular injury that is caused by cytokine storm (resulting in hypoxemia, septic shock, ARDS,and multi-organ failure) by blocking IL-6. Dose is 4 to 8 mg/kg (max 800-mg/dose), up to 2 doses given 12 hours apart.
Lopinavir–Ritonavir: In a randomized, controlled, open-label trialinvolving hospitalized adults with confirmed COVID-19, no benefit was observed with lopinavir–ritonavir treatment beyond standard care. (However, study drug was started late in severely ill patients when cytokine storm is probably the main driver of mortality. Perhaps early use could have improved the outcomes)
Favipiravir: Broad-spectrum anti-viral drug that woks by selective inhibition of viral RNA-dependent RNA polymerase. Approved in 2014 in Japan for stockpiling against influenza pandemics. An open-label control study in China comparing Favipiravir against lopinavir/ritonavir showed shorter viral clearance time and improvement in chest imaging compared to LPV/RTV. (Not available in US)
Convalescent plasma: Patients who recover from COVID-19 have neutralizing antibodies against the virus. So, in theory, pooled IVIG from patients who recover from illness should help. It seems to work for SARS. So it might work for SARS-CoV-2 too.
Steroids: May prolong viral replication (observed in MERS-CoV). Therefore do NOT use steroids to treat COVID-19 related pneumonitis.
Other agents: A number of anti-retrovirals such as Lopinavir/ritonavir, Ribaviran, and Nitazoxanide (1g po b.i.d. x 5days) are curently either being used or studied.
Nearly 70 drugs and experimental compounds may be effective in treating the coronavirus. So stay tuned for a lot of in-vitro and human studies in coming months.
Chloroquine or hydroxychloroquine for COVID-19 prophylaxis
This is a subject of pure speculation at this time. However, since a lot of people are asking about it or considering it, here are some preliminary thoughts:
Could it work? Possibly. See mechanism of action described above in Anti-viral medications section.
Who should be taking it? No one on their own. Physicians may consider it for their heavily immunocompromised patients such as lung transplant recipients, BMT, etc. who are either exposed to a COVID-19 patient or at significant risk of exposure.
Should healthcare workers taking care of COVID-19 patients use it? No data to guide. Your hospital should make a policy about it. I personally think it might be worth considering in healthcare providers taking care of confirmed COIVD-19 cases.
Dose and duration is unknown but two ongoing clinical trials, one for primary prophylaxis and other for secondary (post-exposure) prophylaxis in healthcare setting are investigating it. See trial design for dosing and schedule.
Outside the clinical trials, once weekly dosing may be appropriate. (e.g. Chloroquine 500 mg once weekly) as the drug has very long half-life (over 3 weeks) — personal opinion.
Should general public take it for prevention? No.
Prevention
‘Flattening the curve’ — Source: ourworldindata.org
Mitigation strategies such as social distancing, isolation of known cases at home or in healthcare setting, and other measures to stop the spread the infection can slow the epidemic, termed as “Flattening the curve”, easing the burden on healthcare system and giving more time for preparation.
Read the original CDC paper here.
I have outlined preventive measures for general public in this blog postincluding social distancing, frequent hand washing (with soap and water), covering cough and sneezes, and staying home when sick.
For healthcare workers, follow your institutional and CDC guidelines. Some additional ideas are:
~ Creating a command center to coordinate all COVID-19 planning and communications. If possible, designate a geographic area in the hospital that is well equipped to handle COVID-19 patients. Restrict visitors.
~ Update the FIT testing for all hospital staff taking care of COVID-19patients. Limit number of healthcare providers who see a COVID-19 patient. Utilize available telemedicine tools to reduce exposure while delivering affective care.
‘Flattening the curve’ — Source: ourworldindata.org
March 21, 2020
COVID-19: Understanding the Science, Epidemiology and Clinical Manifestations
On March 19, 2020 during Grand Rounds at the University of California SF, Dr Diane Havlir (epidemiologist and virologist) among other colleagues discussed the science, epidemiology and clinical manifestations of COVID 19. We decided to share with you a quick snap shot of testing sensitivity and specificity.
Current tests are PCR-based, but sensitivity varies significantly by sampling type. Highest specificity is in nasopharynx & oropharynx.
Single NP swab sensitivity is 75%.
Single OP swab is about 33%.
But if you sample at both NP&OP sites, sensitivity is 85%.
Lower respiratory tract sampling is optimal: BAL sample is >90% specificity.
Click on the link below for a full report from the March 19, 2020 UCSF Grand Rounds
March 19, 2020
Speaking on what the Ebola Outbreak in West Africa had taught us, Bill Gates of Microsoft discussed the importance of world preparedness to the next global pandemic in few points back in 2015 TedTalk. At CURA for the world we discussed the importance of integrating NGOs around the world, on April 29th 2019 at the United Nations High Level Meeting on Universal Healthcare. We stated that enriching NGOs with small scale general medicine clinics, such of what CURA for the world is doing, and to link their resources together to further identify early signs of outbreaks through geofencing.
In his talk CURA for the world identifies strongly with his first and third points at the present time. And his other points seems very logical and feasible with the spirit of cooperation.
1. Strengthen health systems
2. Create a medical reserve corps
3. Pair medical and military : logistics and security
4. Simulations: Germ games not war games
5. Step up research and development
March 15, 2020
** THIS POST IS INTENDED FOR HEALTH CARE PROFESSIONALS **
Nomenclature: Coronavirus Disease 2019 a.k.a. COVID - 19
Virus : SARS-CoV-2, 2019 Novel Coronavirus (Not Wuhan Virus)
Biology
Enveloped coronavirus 30 kbp, +ssRNA
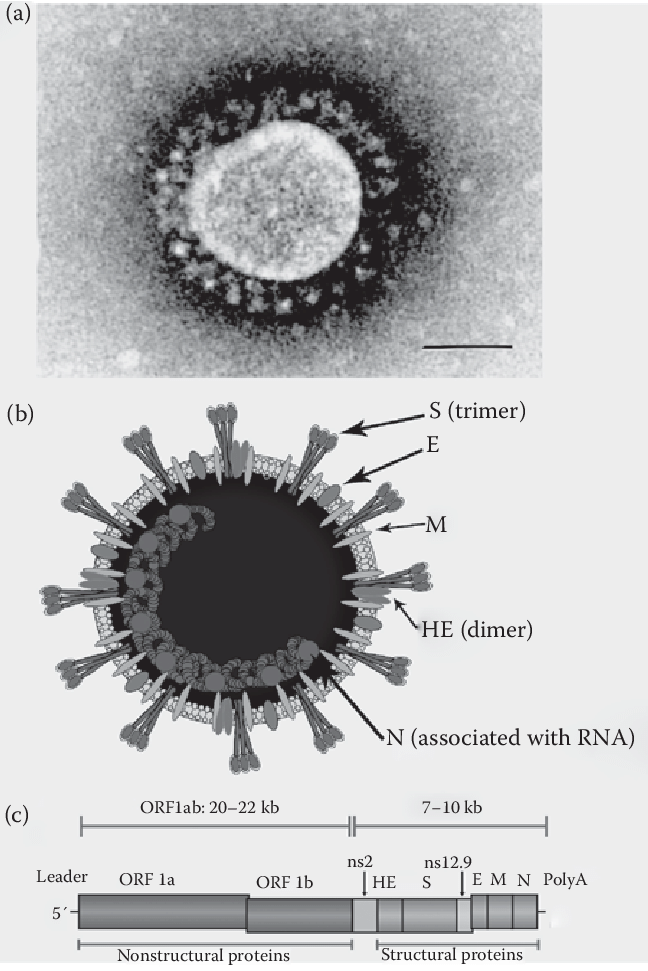
Coronavirus
Overview of the coronaviruses: electron microscopic appearance (a; bar = 100 nm) and schematic diagram of the viral particle (b) and of the 30-kb RNA genome (c). The viral particle and the genome of the β-coronavirus HCoV-OC43 are represented as an example, but the overall organization of all the coronaviruses is the same, with the exception of the HE gene, which encodes a hemagglutinin-esterase protein, only present as a fifth structural protein in the viral envelope of some coronaviruses. The nonstructural (ns) proteins are specific to each genus and their number varies between the different coronaviruses. ORF1 encodes two large polyproteins designated pp1a and pp1ab, which are cleaved by viral proteases to yield 15 to 16 nonstructural proteins (nsp).
Source and Spread of the Virus (Virology)
Source/reservoir is unclear (Bats? Pangolins ? …. eventually to people) - Wet Market in wuhan, china.
Coronaviruses are a large family of viruses that are common in people and many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS-CoV, SARS-CoV, and now with this new virus (named SARS-CoV-2).
The SARS-CoV-2 virus is a betacoronavirus, like MERS-CoV and SARS-CoV. All three of these viruses have their origins in bats. The sequences from U.S. patients are similar to the one that China initially posted, suggesting a likely single, recent emergence of this virus from an animal reservoir.
Early on, many of the patients at the epicenter of the outbreak in Wuhan, Hubei Province, China had some link to a large seafood and live animal market, suggesting animal-to-person spread. Later, a growing number of patients reportedly did not have exposure to animal markets, indicating person-to-person spread. Person-to-person spread was subsequently reported outside Hubei and in countries outside China, including in the United States. Some international destinations now have ongoing community spread with the virus that causes COVID-19, as do some parts of the United States. Community spread means some people have been infected and it is not known how or where they became exposed. Learn what is known about the spread of this newly emerged coronaviruses.
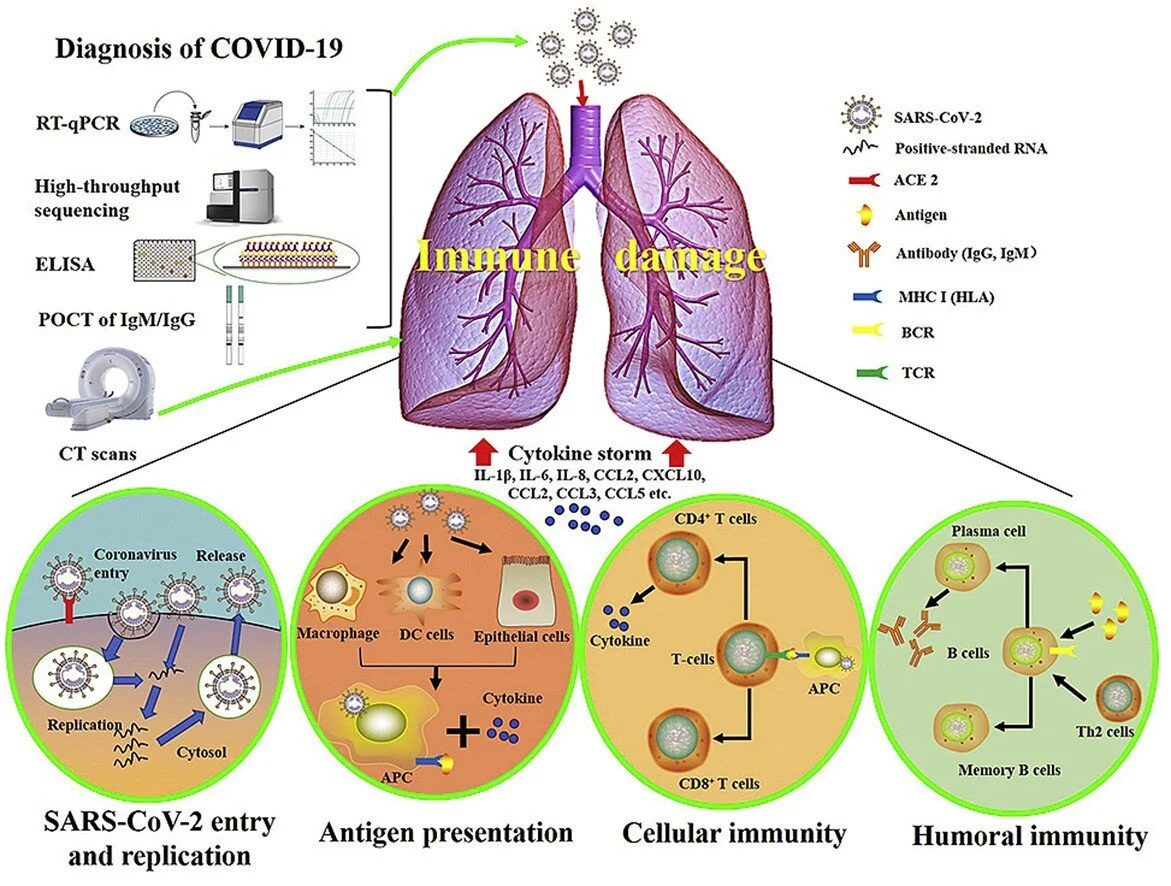
Now it is spread primarily person to person. Virus can be spread by asymptomatic carriers. Viral Particles enter into the lungs via droplets. Viral S spoke binds to ACE-2 on type 2 pneumocystis. Effect of ACE/ARB is unclear and is not recommended to change medications at this time. Other routes of infection include contact and enteric, possible but unclear if these are significant means of spread.
Pathogenesis
SARS-CoV-2 enters cells via ACE-2 receptors which are expressed by epithelial cells of the lung, intestine, kidney, and blood vessels. Initial cellular injury is causes by direct viral Cytopathic effect (anti-viral therapy is likely to help at this stage). Subsequently, additional damage is caused by cytokine storm (Immunomodulation therapy, such as IL-6 blockers, are likely to help at this stage). To learn more, read this chapteron Advances in Virus Research.
Source: Li et al.
Epidemiology
Geographic distribution — Since the first reports of cases from Wuhan, a city in the Hubei Province of China, at the end of 2019, more than 80,000 COVID-19 cases have been reported in China; these include all laboratory-confirmed cases as well as clinically diagnosed cases in the Hubei Province. A joint World Health Organization (WHO)-China fact-finding mission estimated that the epidemic in China peaked between late January and early February 2020 [4]. The majority of reports have been from Hubei and surrounding provinces, but numerous cases have been reported in other provinces and municipalities throughout China [5,6].
Attack rate = 30-40%
Ro = 2-4
Case Fatality Rate (CFR) = 3-6% (worldwide numbers)
Increasing numbers of cases have also been reported in other countries across all continents except Antarctica, and the rate of new cases outside of China has outpaced the rate in China. These cases initially occurred mainly among travelers from China and those who have had contact with travelers from China [7-11]. However, ongoing local transmission has driven smaller outbreaks in some locations outside of China, including South Korea, Italy, Iran, and Japan, and infections elsewhere have been identified in travelers from those countries [12].
In the United States, several clusters of COVID-19 with local transmission have been identified throughout the country.
Updated case counts in English can be found on the World Health Organization and European Centre for Disease Prevention and Control websites.
TIMELINE
China Notifies WHO 12-31-2019
First US case in Seattle 2-15-2020
WHO declares pandemic 3-11-2020
Declaration of National Emergency 3-12-2020
CLINICAL FEATURES
Incubation period — The incubation period for COVID-19 is thought to be within 14 days following exposure, with most cases occurring approximately four to five days after exposure [32-34].
In a study of 1099 patients with confirmed symptomatic COVID-19, the median incubation period was four days (interquartile range two to seven days) [33].
Using data from 181 publicly reported, confirmed cases in China with identifiable exposure, one modeling study estimated that symptoms would develop in 2.5 percent of infected individuals within 2.2 days and in 97.5 percent of infected individuals within 11.5 days [35]. The median incubation period in this study was 5.1 days.
VIRAL SHEDDING: median 20 days (max 37 days)
Spectrum of illness severity
Most infections are not severe, although many patients with COVID-19 have critical illness [34,36-41]. Specifically, in a report from the Chinese Center for Disease Control and Prevention that included approximately 44,500 confirmed infections with an estimation of disease severity [42]:
●Mild (no or mild pneumonia) was reported in 81 percent.
●Severe disease (eg, with dyspnea, hypoxia, or >50 percent lung involvement on imaging within 24 to 48 hours) was reported in 14 percent.
●Critical disease (eg, with respiratory failure, shock, or multiorgan dysfunction) was reported in 5 percent.
●The overall case fatality rate was 2.3 percent; no deaths were reported among noncritical cases.
According to a joint World Health Organization (WHO)-China fact-finding mission, the case-fatality rate ranged from 5.8 percent in Wuhan to 0.7 percent in the rest of China [15]. Most of the fatal cases have occurred in patients with advanced age or underlying medical comorbidities.
Age range
Individuals of any age can acquire severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, although adults of middle age and older are most commonly affected.
In several cohorts of hospitalized patients with confirmed COVID-19, the median age ranged from 49 to 56 years [37-39]. In a report from the Chinese Center for Disease Control and Prevention that included approximately 44,500 confirmed infections, 87 percent of patients were between 30 and 79 years old [42]. Older age was also associated with increased mortality, with a case fatality rate of 8 and 15 percent among those aged 70 to 79 years and 80 years or older, respectively.
Symptomatic infection in children appears to be uncommon; when it occurs, it is usually mild, although severe cases have been reported. In the large Chinese report described above, only 2 percent of infections were in individuals younger than 20 years old [42]. In a small study of 10 children, clinical illness was mild; 8 had fever, which resolved within 24 hours, 6 had cough, 4 had sore throat, 4 had evidence of focal pneumonia on CT, and none required supplemental oxygen [43]. In another study of six children aged 1 to 7 years who were hospitalized in Wuhan with COVID-19, all had fever >102.2°F/39°C and cough, four had imaging evidence of viral pneumonia, and one was admitted to the intensive care unit; all children recovered [44].
Asymptomatic infections
Asymptomatic infections have also been described [34,45-47], but their frequency is unknown.
In a COVID-19 outbreak on a cruise ship where nearly all passengers and staff were screened for SARS-CoV-2, approximately 17 percent of the population on board tested positive as of February 20; about half of the 619 confirmed COVID-19 cases were asymptomatic at the time of diagnosis [48].
Even patients with asymptomatic infection may have objective clinical abnormalities. In another study of 24 patients with asymptomatic infection who all underwent chest computed tomography (CT), 50 percent had typical ground-glass opacities or patchy shadowing, and another 20 percent had atypical imaging abnormalities [21]. Five patients developed low-grade fever, with or without other typical symptoms, a few days after diagnosis.
Clinical presentation
Pneumonia appears to be the most frequent serious manifestation of infection, characterized primarily by fever, cough, dyspnea, and bilateral infiltrates on chest imaging [33,37-39]. There are no specific clinical features that can yet reliably distinguish COVID-19 from other viral respiratory infections.
In a study describing 138 patients with COVID-19 pneumonia in Wuhan, the most common clinical features at the onset of illness were [39]:
●Fever in 99 percent
●Fatigue in 70 percent
●Dry cough in 59 percent
●Anorexia in 40 percent
●Myalgias in 35 percent
●Dyspnea in 31 percent
●Sputum production in 27 percent
The dyspnea developed after a median of five days of illness. Acute respiratory distress syndrome developed in 20 percent, and mechanical ventilation was implemented in 12.3 percent.
Other cohort studies of patients from Wuhan with confirmed COVID-19 have reported a similar range of clinical findings [37,39,49,50]. However, fever might not be a universal finding. In one study, fever was reported in almost all patients, but approximately 20 percent had a very low grade fever <100.4°F/38°C [37]. In another study of 1099 patients from Wuhan and other areas in China, fever (defined as an axillary temperature over 99.5°F/37.5°C) was present in only 44 percent on admission but was ultimately noted in 89 percent during the hospitalization [33].
Other, less common symptoms have included headache, sore throat, and rhinorrhea. In addition to respiratory symptoms, gastrointestinal symptoms (eg, nausea and diarrhea) have also been reported in some patients, but these are relatively uncommon [37,39].
Reports of cohorts in locations outside of Wuhan have described similar clinical findings, although some have suggested that milder illness may be more common [51-53]. As an example, in a study of 62 patients with COVID-19 in the Zhejiang province of China, all but one had pneumonia, but only two developed dyspnea, and only one warranted mechanical ventilation [52].
According to the WHO, recovery time appears to be around two weeks for mild infections and three to six weeks for severe disease [4].
Laboratory findings
In patients with COVID-19, the white blood cell count can vary.
CBC : Leukopenia, leukocytosis, and lymphopenia (80%) have been reported
BMP: Increased BUN and Cr , Elevated aminotransferase levels have also been described increase AST, ALT and Tbili
On admission, many patients with pneumonia have normal serum procalcitonin PCT levels; however, in those requiring intensive care unit (ICU) care, they are more likely to be elevated [37-39].
In one study, high D-dimer levels and more severe lymphopenia were associated with mortality [38].
Increased CRP, LDH
Increased IL-6 and Ferritin.
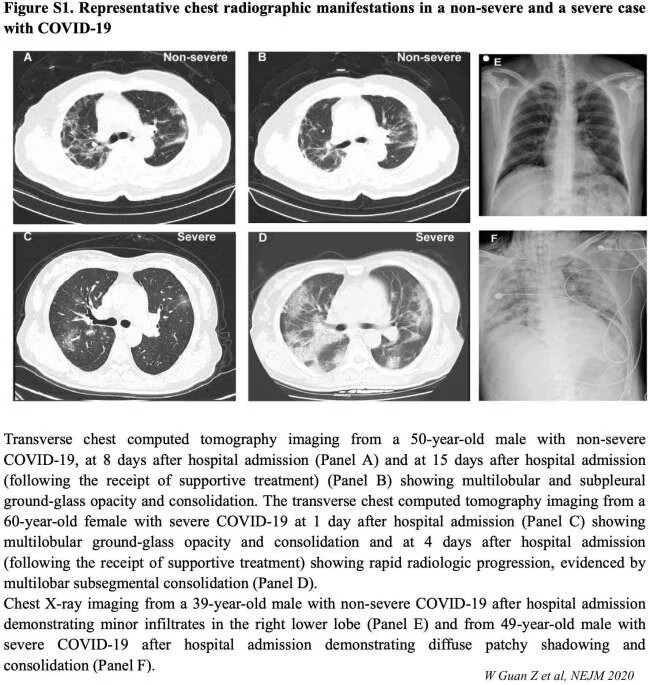
Imaging findings
Chest CT in patients with COVID-19 most commonly demonstrates ground-glass opacification with or without consolidative abnormalities, consistent with viral pneumonia [50,54]. Case series have suggested that chest CT abnormalities are more likely to be bilateral, have a peripheral distribution, and involve the lower lobes. Less common findings include pleural thickening, pleural effusion, and lymphadenopathy.
Chest CT may be helpful in making the diagnosis, but no finding can completely rule in or rule out the possibility of COVID-19. In a study of 1014 patients in Wuhan who underwent both reverse-transcription polymerase chain reaction (RT-PCR) testing and chest CT for evaluation of COVID-19, a "positive" chest CT for COVID-19 (as determined by a consensus of two radiologists) had a sensitivity of 97 percent, using the PCR tests as a reference; however, specificity was only 25 percent [55]. The low specificity may be related to other etiologies causing similar CT findings. In another study comparing chest CTs from 219 patients with COVID-19 in China and 205 patients with other causes of viral pneumonia in the United States, COVID-19 cases were more likely to have a peripheral distribution (80 versus 57 percent), ground-glass opacities (91 versus 68 percent), fine reticular opacities (56 versus 22 percent), vascular thickening (59 versus 22 percent), and reverse halo sign (11 versus 1 percent), but less likely to have a central and peripheral distribution (14 versus 35 percent), air bronchogram (14 versus 23 percent), pleural thickening (15 versus 33 percent), pleural effusion (4 versus 39 percent), and lymphadenopathy (2.7 versus 10 percent) [56]. A group of radiologists in that study was able to distinguish COVID-19 with high specificity but moderate sensitivity.
In one report of 21 patients with laboratory-confirmed COVID-19 who did not develop severe respiratory distress, lung abnormalities on chest imaging were most severe approximately 10 days after symptom onset [49]. However, chest CT abnormalities have also been identified in patients prior to the development of symptoms and even prior to the detection of viral RNA from upper respiratory specimens [50,57].
EVALUATION AND DIAGNOSIS
Clinical suspicion and criteria for testing
The approach to initial management should focus on early recognition of suspect cases, immediate isolation, and institution of infection control measures. At present, the possibility of COVID-19 should be considered primarily in patients with fever and/or lower respiratory tract symptoms who have had any of the following in the prior 14 days:
●Close contact with a confirmed or suspected case of COVID-19, including through work in health care settings. Close contact includes being within approximately six feet (about two meters) of a patient for a prolonged period of time while not wearing personal protective equipment or having direct contact with infectious secretions while not wearing personal protective equipment.
●Residence in or travel to areas where widespread community transmission has been reported (eg, China, South Korea, most of Europe [including Italy], Iran, Japan). (See 'Geographic distribution' above.)
●Potential exposure through attendance at events or spending time in specific settings where COVID-19 cases have been reported.
The possibility of COVID-19 should also be considered in patients with severe lower respiratory tract illness when an alternative etiology cannot be identified, even if there has been no clear exposure.
When COVID-19 is suspected, infection control measures should be implemented and public health officials notified. Patients who do not need emergent care should be encouraged to call prior to presenting to a health care facility for evaluation. Many patients can be evaluated regarding the need for testing over the phone. Infection control precautions are discussed elsewhere. (See 'Infection control for suspected or confirmed cases' below.)
The specific case definitions and clinical criteria for pursuing diagnostic evaluation differ slightly between expert groups.
●The United States Centers for Disease Control and Prevention (CDC) notes that the decision to test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) should be based on clinical judgment and reminds clinicians that most patients with confirmed COVID-19 have fever (subjective or confirmed) and/or symptoms of acute respiratory illness (eg cough, dyspnea). This guidance expands its previous criteria to potentially include a wider group of symptomatic patients. In areas where testing capacity is limited, public health officials can guide prioritization of testing. The CDC suggests prioritizing hospitalized patients to inform infection control decisions, symptomatic individuals who have a higher risk of poor outcomes (eg, age ≥65 years, chronic medical condition, immunocompromising conditions), and those with high exposure risk (eg, recent travel to specific locations, contact with patients with COVID-19, or being a health care worker) [58]. Details can be found on the CDC website. An approach to suspected cases when testing is not available is discussed elsewhere. (See 'COVID-19 testing not readily available' below.)
●Case definitions from the World Health Organization are found in its technical guidance online.
●Case definitions from the European Centre for Disease Prevention and Control are found on its website.
Laboratory testing
Patients who meet the criteria for suspect cases, as discussed above, should undergo testing for SARS-CoV-2 (the virus that causes COVID-19), in addition to testing for other respiratory pathogens. (See "Diagnostic approach to community-acquired pneumonia in adults", section on 'Diagnostic testing for microbial etiology'.)
In the United States, the CDC recommends collection of specimens to test for SARS-CoV-2 from the upper respiratory tract (nasopharyngeal and oropharyngeal swab) and, if possible, the lower respiratory tract (sputum, tracheal aspirate, or bronchoalveolar lavage) [59]. Induction of sputum is not indicated. Additional specimens (eg, stool, urine) can also be collected. Respiratory specimen collection should be performed under airborne precautions.
SARS-CoV-2 RNA is detected by polymerase chain reaction; in the United States, testing is performed by the CDC or a CDC-qualified lab [60]. A positive test for SARS-CoV-2 confirms the diagnosis of COVID-19. If initial testing is negative but the suspicion for COVID-19 remains, the WHO recommends resampling and testing from multiple respiratory tract sites [61]. Negative reverse-transcription polymerase chain reaction (RT-PCR) tests on oropharyngeal swabs despite CT findings suggestive of viral pneumonia have been reported in some patients who ultimately tested positive for SARS-CoV-2 [57].
For safety reasons, specimens from a patient with suspected or documented COVID-19 should not be submitted for viral culture.
PCR TEST
The importance of testing for other pathogens was highlighted in a report of 210 symptomatic patients with suspected COVID-19; 30 tested positive for another respiratory viral pathogen, and 11 tested positive for SARS-CoV-2 [36].
example of a home test
MANAGEMENT
Home care
Home management is appropriate for patients with mild infection who can be adequately isolated in the outpatient setting [23,62,63]. Management of such patients should focus on prevention of transmission to others, and monitoring for clinical deterioration, which should prompt hospitalization.
Outpatients with COVID-19 should stay at home and try to separate themselves from other people and animals in the household. They should wear a facemask when in the same room (or vehicle) as other people and when presenting to health care settings. United States Centers for Disease Control and Prevention (CDC) recommendations on discontinuation of home isolation are discussed below. (See 'Discontinuation of precautions' below.)
More detailed interim recommendations on home management of patients with COVID-19 can be found on the WHO and CDCwebsites [63-65].
Hospital care
Some patients with suspected or documented COVID-19 have severe disease that warrants hospital care. Management of such patients consists of ensuring appropriate infection control, as below (see 'Infection control for suspected or confirmed cases' below), and supportive care. Clinical guidance can be found on the World Health Organization (WHO) and CDC websites [23,62].
Patients with severe disease often need oxygenation support. High-flow oxygen and noninvasive positive pressure ventilation have been used, but the safety of these measures is uncertain, and they should be considered aerosol-generating procedures that warrant specific isolation precautions . (See 'Infection control for suspected or confirmed cases' below.)
Some patients may develop acute respiratory distress syndrome and warrant intubation with mechanical ventilation; extracorporeal membrane oxygenation may be indicated in patients with refractory hypoxia. Management of acute respiratory distress syndrome is discussed in detail elsewhere. (See "Evaluation and management of suspected sepsis and septic shock in adults" and "Acute respiratory distress syndrome: Supportive care and oxygenation in adults".)
The WHO and CDC recommend glucocorticoids not be used in patients with COVID-19 pneumonia unless there are other indications (eg, exacerbation of chronic obstructive pulmonary disease) [23,62]. Glucocorticoids have been associated with an increased risk for mortality in patients with influenza and delayed viral clearance in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Although they were widely used in management of severe acute respiratory syndrome (SARS), there was no good evidence for benefit, and there was persuasive evidence of adverse short- and long-term harm [66]. (See "Treatment of seasonal influenza in adults", section on 'Adjunctive therapies' and "Middle East respiratory syndrome coronavirus: Treatment and prevention", section on 'Treatment'.)
Investigational agents are being explored for antiviral treatment of COVID-19, and enrollment in clinical trials should be discussed with patients or their proxies. A registry of international clinical trials can be found on the WHO website and at clinicaltrials.gov.
Several randomized trials are underway to evaluate the efficacy of remdesivir for moderate or severe COVID-19 [67]. Remdesivir is a novel nucleotide analogue that has activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro and related coronaviruses (including SARS and MERS-CoV) both in vitro and in animal studies [68,69]. The compassionate use of remdesivir through an investigational new drug application was described in a case report of one of the first patients with COVID-19 in the United States [70]. Any clinical impact of remdesivir on COVID-19 remains unknown.
There has also been interest in the combined protease inhibitor lopinavir-ritonavir, which is used for the treatment of HIV infection. This combined agent has in vitro activity against the SARS-CoV [71] and appears to have some activity against MERS-CoV in animal studies [72]. The use of this agent for treatment of COVID-19 has been described in case reports [73-75], but its efficacy is unclear, and it is being evaluated in larger randomized trials.
Other interventions of interest but with limited or no clinical data include chloroquine [76], hydroxychloroquine [77], interferon beta, and convalescent serum.
Practitioners should be aware of local guidelines regarding treatment and also assess their patients for eligibility in available clinical trials. Treatment guidelines from China's National Health Commission include the IL-6 inhibitor tocilizumab for severe infection, although clinical data are limited; the agent is being evaluated in a clinical trial [78].
PREVENTION
In the health care setting
Screening and precautions for fever or respiratory symptoms — Screening patients for clinical manifestations consistent with COVID-19 (eg, fever, cough, dyspnea) prior to entry into a health care facility can help identify those who may warrant additional infection control precautions. This can be done over the phone before the patient actually presents to a facility. Any individual with these manifestations should be advised to wear a facemask. Separate waiting areas for patients with respiratory symptoms should be designated, if possible, at least six feet away from the regular waiting areas.
Symptomatic patients should also be asked about recent travel or potential COVID-19 exposure in the prior 14 days to determine the need for evaluation for COVID-19. (See 'Clinical suspicion and criteria for testing' above.)
In some settings, such as long-term care facilities, the United States Centers for Disease Control and Prevention (CDC) recommends that standard, contact, and droplet precautions in addition to eye protection be used for any patient with an undiagnosed respiratory infection who is not under consideration for COVID-19 [79]. This may help reduce the risk of spread from unsuspected COVID-19 cases. Infection control precautions for suspect COVID-19 cases are discussed below.
In locations where community transmission is ongoing, postponing elective procedures or non-urgent visits and using virtual (eg, through video communication) visits may be useful strategies to reduce the risk of exposure [80].
Infection control for suspected or confirmed cases — Infection control to limit transmission is an essential component of care in patients with suspected or documented COVID-19. In one report of 138 patients with COVID-19 in China, it was estimated that 43 percent acquired infection in the hospital setting [39].
Individuals with suspected infection in the community should be advised to wear a medical mask to contain their respiratory secretions prior to seeking medical attention. (See 'Evaluation and diagnosis' above.)
In the health care setting, the World Health Organization (WHO) and CDC recommendations for infection control for suspected or confirmed infections differ slightly:
●The WHO recommends standard, contact, and droplet precautions (ie, gown, gloves, and mask), with eye or face protection [81]. The addition of airborne precautions (ie, respirator) is warranted during aerosol-generating procedures (as detailed below).
The CDC recommends that patients with suspected or confirmed COVID-19 be placed in a single-occupancy room with a closed door and dedicated bathroom [80]. The patient should wear a facemask if being transported out of the room (eg, for studies that cannot be performed in the room). An airborne infection isolation room (ie, a single-patient negative pressure room) should be reserved for patients undergoing aerosol-generating procedures (as detailed below).
Any personnel entering the room of a patient with suspected or confirmed COVID-19 should wear the appropriate personal protection equipment: gown, gloves, eye protection, and a respirator (eg, an N95 respirator). If supply of respirators is limited, the CDC acknowledges that facemasks are an acceptable alternative (in addition to contact precautions and eye protection), but respirators should be worn during aerosol-generating procedures [80].
Aerosol-generating procedures include tracheal intubation, noninvasive ventilation, tracheotomy, cardiopulmonary resuscitation, manual ventilation before intubation, and bronchoscopy. Nasopharyngeal or oropharyngeal specimen collection is not considered an aerosol-generating procedure.
For health care workers who have had a potential exposure to COVID-19, the CDC has provided guidelines for work restriction and monitoring. The approach depends upon the duration of exposure, the patient's symptoms, whether the patient was wearing a facemask, the type of personal protective equipment used by the provider, and whether an aerosol-generating procedure was performed.
Links to additional infection control guidelines are found below. (See 'Society guideline links' below.)
Discontinuation of precautions
The decision to discontinue infection control precautions for patients with COVID-19 should be made on a case-by-case basis in consultation with experts in infection prevention and control and public health officials. Factors to inform this decision include resolution of clinical signs and symptoms and negative results of reverse-transcription polymerase chain reaction (RT-PCR) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on two sequential paired nasopharyngeal and throat specimens (ie, four specimens total, each handled separately), with each pair collected ≥24 hours apart [82].
Positive RT-PCR tests for SARS-CoV-2 were reported in four laboratory-confirmed COVID-19 patients after they had clinically improved and tested negative on two consecutive tests [83]. The clinical significance of this finding is uncertain; it is unknown whether these individuals continued to shed infectious virus.
Environmental disinfection
To help reduce the spread of COVID-19 virus, environmental infection control procedures should also be implemented [63,65,80,81,84]. In United States health care settings, the CDC states routine cleaning and disinfection procedures are appropriate for COVID-19 virus [80].
Products approved by the Environmental Protection Agency (EPA) for emerging viral pathogens should be used; a list of EPA-registered products can be found here. Specific guidance on environmental measures, including those used in the home setting, is available on the CDC and WHO websites. Additional information is also found in a separate topic review. (See "Coronaviruses", section on 'Treatment and prevention'.)
The importance of environmental disinfection was illustrated in a study from Singapore, in which viral RNA was detected on nearly all surfaces tested (handles, light switches, bed and handrails, interior doors and windows, toilet bowl, sink basin) in the airborne infection isolation room of a patient with symptomatic mild COVID-19 prior to routine cleaning [85]. Viral RNA was not detected on similar surfaces in the rooms of two other symptomatic patients following routine cleaning (with sodium dichloroisocyanurate). Of note, viral RNA detection does not necessarily indicate the presence of infectious virus.
It is unknown how long SARS-CoV-2 can persist on surfaces; other coronaviruses have been tested and may survive on inanimate surfaces for up to six to nine days without disinfection. In a study evaluating the survival of viruses dried on a plastic surface at room temperature, a specimen containing SARS-CoV (a virus closely related to SARS-CoV-2) had detectable infectivity at six but not nine days [86]. However, in a systematic review of similar studies, various disinfectants (including ethanol at concentrations between 62 and 71 percent) inactivated a number of coronaviruses related to SARS-CoV-2 within one minute [84].
Preventing exposure in the community
The following general measures are recommended to reduce transmission of infection:
●Diligent hand washing, particularly after touching surfaces in public. Use of hand sanitizer that contains at least 60 percent alcohol is a reasonable alternative if the hands are not visibly dirty.
●Respiratory hygiene (eg, covering the cough or sneeze).
●Avoiding touching the face (in particular eyes, nose, and mouth).
●Avoiding crowds (particularly in poorly ventilated spaces) if possible and avoiding close contact with ill individuals.
●Cleaning and disinfecting objects and surfaces that are frequently touched. The CDC has issued guidance on disinfection in the home setting; a list of EPA-registered products can be found here.
In particular, older adults and individuals with chronic medical conditions should be encouraged to follow these measures.
If SARS-CoV-2 is prevalent in the community, residents should be encouraged to practice social distancing by staying home as much as possible.
For people without respiratory symptoms, wearing a medical mask in the community is not recommended, even if COVID-19 is prevalent in the area [2]; wearing a mask does not decrease the importance of other general measures to prevent infection, and it may result in unnecessary cost and supply problems [87].
Individuals who are caring for patients with suspected or documented COVID-19 at home, however, should wear a tightly fitting medical mask when in the same room as that patient.
Individuals who develop an acute respiratory illness (eg, with fever and/or respiratory symptoms) should be encouraged to stay home from school or work for the duration of the illness. Some may warrant evaluation for COVID-19. (See 'Clinical suspicion and criteria for testing' above.)
The CDC has included recommended measures to prevent spread in the community on its website.
Managing asymptomatic individuals with potential exposure
Individuals who have had travel to high-risk areas or are contacts of patients with suspected or confirmed COVID-19 should be monitored for development of consistent symptoms and signs (fever, cough, or dyspnea). Such clinical manifestations should prompt at least self-isolation with social distancing and clinician assessment for the need for medical evaluation. (See 'Clinical suspicion and criteria for testing' above.)
In the United States, the level of risk (based on the travel location or the type of contact) informs whether monitoring and isolation are done by the individual or with the involvement of public health personnel. Categories of risk and the suggested monitoring and isolation strategies can be found on the CDC website.
Global public health measures
On January 30, 2020, the WHO declared the COVID-19 outbreak a public health emergency of international concern and, in March 2020, began to characterize it as a pandemic in order to emphasize the gravity of the situation and urge all countries to take action in detecting infection and preventing spread. The WHO has indicated three priorities for countries: protecting health workers, engaging communities to protect those at highest risk of severe disease (eg, older adults and those with medical comorbidities), and supporting vulnerable countries in containing infection [4].
The WHO does not recommend international travel restrictions but does acknowledge that movement restriction may be temporarily useful in some settings. The WHO advises exit screening for international travelers from areas with ongoing transmission of COVID-19 virus to identify individuals with fever, cough, or potential high-risk exposure [88,89]. Many countries also perform entry screening (eg, temperature, assessment for signs and symptoms). More detailed travel information is available on the WHO website.
In the United States, the CDC currently recommends that individuals avoid all nonessential travel to mainland China, Iran, most European countries (including Italy), and South Korea [90]. Because risk of travel changes rapidly, those coming from other countries should check United States government web sites for possible restrictions on arrival. The CDC has released travel advisories regarding other locations where community transmission has been reported [90]. The CDC website provides updated guidance on travel restrictions as well as risk assessment and management of persons with a suspected exposure to COVID-19.
Although many cases of COVID-19 can be detected through entry screening, some may be missed. As an example, in Germany, 114 travellers returning from Wuhan were considered to be asymptomatic during entry screening but, when tested for COVID-19 virus by RT-PCR, two tested positive [91]. However, the role of asymptomatic patients in transmitting infection to others, and thus the value of PCR testing of asymptomatic individuals on entry, remains unclear. (See 'Transmission' above.)
SPECIAL SITUATIONS
Pregnant women
Minimal information is available regarding COVID-19 during pregnancy. Intrauterine or perinatal transmission has not been identified [92-95]. In two reports including a total of 18 pregnant women with suspected or confirmed COVID-19 pneumonia, there was no laboratory evidence of transmission of the virus to the neonate [92,93]. However, two neonatal cases of infection have been documented [96]. In one case, the diagnosis was made at day 17 of life after close contact with the infant's mother and a maternity matron who were both infected with the virus. The other case was diagnosed 36 hours after birth; the source and time of transmission in that case were unclear.
The approach to prevention, evaluation, diagnosis, and treatment of pregnant women with suspected COVID-19 should be similar to that in nonpregnant individuals (as described above), with consideration that pregnant women with other potentially severe respiratory infections, such as influenza, severe acute respiratory syndrome (SARS)-CoV, or Middle East respiratory syndrome (MERS)-CoV, appear to be more vulnerable to developing severe disease.
Additionally, the American College of Obstetricians and Gynecologists (ACOG) specifies that infants born to mothers with confirmed COVID-19 should be considered a patient under investigation and appropriately isolated and evaluated [97]. (See 'Evaluation and diagnosis' above.)
It is unknown whether the virus can be transmitted through breast milk; however, droplet transmission could occur through close contact during breastfeeding. ACOG recommends that mothers with confirmed COVID-19 or symptomatic mothers with suspected COVID-19 take precautions to prevent transmission to the infant during breastfeeding (including assiduous hand hygiene and using a facemask) or consider having a different individual feed expressed breast milk to the infant [97].
COVID-19 testing not readily available ? (Some online platforms can provide some testing)
In some cases, testing for COVID-19 may not be accessible, particularly for individuals who have a compatible but mild illness that does not warrant hospitalization and do not have a known COVID-19 exposure or high-risk travel history.
In the United States, there is limited official guidance for this situation, and the approach may depend on the prevalence of COVID-19 in the area. If the clinician has sufficient concern for possible COVID-19 (eg, there is community transmission), it is reasonable to advise the patient to self-isolate at home (if hospitalization is not warranted) and alert the clinician about worsening symptoms. The optimal duration of home isolation in such cases is uncertain. Clinicians should contact their local public health department for guidance. In the state of Washington, the Department of Public Health suggests that individuals with compatible symptoms without exposure to a diagnosed case should continue home isolation until 72 hours after fever and symptoms have resolved [98].
SOCIETY GUIDELINE LINKS
Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Coronavirus disease 2019 (COVID-19)".)
INFORMATION FOR PATIENTS
UpToDate offers two types of patient education materials, "The Basics" and "Beyond the Basics." The Basics patient education pieces are written in plain language, at the 5th to 6th grade reading level, and they answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10th to 12th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon.
Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients. (You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword(s) of interest.)
●Basics topic (see "Patient education: Coronavirus disease 2019 (COVID-19) (The Basics)")
SUMMARY AND RECOMMENDATIONS
●In late 2019, a novel coronavirus, now designated SARS-CoV-2, was identified as the cause of an outbreak of acute respiratory illness in Wuhan, a city in China. In February 2020, the World Health Organization (WHO) designated the disease COVID-19, which stands for coronavirus disease 2019. (See 'Introduction' above.)
●Since the first reports of COVID-19, infection has spread to include more than 80,000 cases in China and increasing cases worldwide, prompting the WHO to declare a public health emergency in late January 2020 and characterize it as a pandemic in March 2020. The rate of new infections outside of China has surpassed that within China as epidemics have grown in other countries. (See 'Epidemiology' above.)
●The possibility of COVID-19 should be considered primarily in patients with fever and/or lower respiratory tract symptoms who have had recent close contact with a confirmed or suspected case of COVID-19, who reside in or have recently (within the prior 14 days) traveled to areas where community transmission has been reported (eg, China, South Korea, most of Europe [including Italy], Iran, Japan) or who have had potential exposure from specific settings where COVID-19 cases have been reported. Clinicians should also be aware of the possibility of COVID-19 in patients with severe respiratory illness when no other etiology can be identified. (See 'Clinical features' above and 'Evaluation and diagnosis'above.)
●Upon suspicion of COVID-19, infection control measures should be implemented and public health officials notified. In health care settings in the United States, the Centers for Disease Control and Prevention (CDC) recommends a single-occupancy room for patients and gown, gloves, eye protection, and a respirator (or facemask as an alternative) for health care personnel. (See 'Infection control for suspected or confirmed cases' above.)
●In addition to testing for other respiratory pathogens, upper respiratory tract (nasopharyngeal and oropharyngeal swab) specimens and, if possible, lower respiratory tract specimens should be tested for SARS-CoV-2. (See 'Evaluation and diagnosis' above.)
●Management consists of supportive care. Home management may be possible for patients with mild illness who can be adequately isolated in the outpatient setting. (See 'Management' above.)
●To reduce the risk of transmission in the community, individuals should be advised to wash hands diligently, practice respiratory hygiene (eg, cover their cough), and avoid crowds and close contact with ill individuals, if possible. Facemasks are not routinely recommended for asymptomatic individuals to prevent exposure in the community. Social distancing is advised in locations that have community transmission. (See 'Preventing exposure in the community' above.)
●Interim guidance has been issued by the WHO and by the CDC. These are updated on an ongoing basis. (See 'Society guideline links' above.)
March 13, 2020
Situation as of 13 March 2020 (10:00am EST)
Of the 23 countries/territories reporting cases in the region of the Americas up to yesterday, 11 of them reported an additional 441 cases of which, the largest proportion was attributable to the United States (361 cases). Trinidad and Tobago and the Cayman Islands reported their first confirmed cases of COVID-19.
The case in Trinidad and Tobago (1) was imported from Switzerland. The case in the Cayman Islands (1) was a patient aboard the “Princess Cruise” who suffered a cardiac arrest and was airlifted onto the island from the ship – he was confirmed for COVID-19.
March 1, 2020
Frequently Asked Questions related to COVID-19 (formerly known as Novel coronavirus 2019)
**Please note that the FAQ’s are subject to change as more information is available.
Q: What does novel mean?
A: A novel coronavirus is a new coronavirus that has not been previously identified.
Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS, SARS, and now with COVID-19.
Q: What are the symptoms and complications that Novel Coronavirus 2019 can cause?
A: Current symptoms reported for patients with COVID-19 have included mild to severe
respiratory illness with fever, cough, and difficulty breathing. Symptoms may appear 2-14 days after exposure. If your patient has been in China, Iran, Italy, South Korea or Japan or has been in known contact with a COVID-19 patient within the past two weeks and develop symptoms, please place a mask on the patient and notify a provider. Travel history is important in identifying the risk for the patient. Areas of concern in the United States are California, Washington and Oregon.
Q: How does the virus spread?
A: This virus probably originally emerged from an animal source but now seems to be spreading from person-to-person. It’s important to note that person-to-person spread can happen on a continuum. Some viruses are highly contagious (like measles), while other viruses are less so. It’s not clear yet how easily COVID-19 spreads from person-to-person. When person-to-person spread has occurred with MERS and SARS, it is thought to have happened mainly via respiratory droplets produced when an infected person coughs or sneezes, similar to how influenza and other respiratory pathogens spread. Spread of MERS and SARS between people has generally occurred between close contacts.
Close contact is defined as:
a) being within approximately 6 feet (2 meters), or within the room or care area, of a novel coronavirus case for a prolonged period of time while not wearing recommended personal protective equipment or PPE (e.g., gowns, gloves, PAPR); close contact can include caring for, living with, visiting, or sharing a health care waiting area or room with a novel coronavirus case
Q: What precautions should healthcare personnel take with a suspected or confirmed COVID-19?
A: Standard precautions should be followed in addition to the following:
· A surgical mask should be placed on the patient as soon as they are identified and to be evaluated in a private room with the door closed, ideally in a negative pressure room (airborne infection isolation room AIIR).
· Healthcare personnel entering the room should use contact precautions, airborne precautions and use eye protection (goggles/face shield).
· Health care providers should consider eye protection, N95 respirator, gloves and a gown. To learn more about donning and doffing please refer to Donning and Doffing policy and poster in the Infection Prevention and Control manual on the library.
Q: If a patient is suspected and needs to be tested what do I do?
A: Healthcare providers should immediately notify both their State Department of Health (Ex. Oklahoma is 405-271-4060 available 24/7) and infection prevention at their facility in the event of a person under investigation (PUI) for COVID-19.
Q: What if a patient suspected to have COVID- 19 requires admission
A: If a patient is admitted to the hospital, he or she will be placed in a negative pressure, isolation room. Maxair PAPRS, isolation gowns and gloves should be worn by all who enter the room. Visitation will be monitored and altered for PUIs.
PUIs with mild to moderate illness, that do not meet admission criteria should be instructed to self-quarantine at home until testing is resulted. The State will monitor their compliance.
Testing for other respiratory pathogens should not delay specimen shipping to SDH/CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI. This may evolve as more information becomes available on possible COVID-19 co-infections
Q: What environmental cleaning methods are available?
A: Example : The PDI Super Sani wipe (purple top) is effective against coronavirus. All commercial cleaners are effective against Coronavirus.
Q: Are there any travel restrictions in place for then general public in the US?
A: Travel to level 2 and 3 countries (China, Iran, Italy, South Korea, Japan) is restricted by the federal government for U.S. nationals. If you chose to travel internationally or to areas of high concern within the U.S. you may be asked to self-quarantine and not return
To see humanity, to reach the unreachable, and find one another. To heal, nourish, and embrace the neglected.